Research
Overview
Our work conbributes to various areas of physical chemistry, in particular to polymer science, colloidal chemistry and nanomaterials, as well as to soft matter physics.
Matthias Ballauff himself is particularly known for having developed new catalyst materials in the form of functionalized metallic nanoparticles dispersed in liquid phase, which can greatly speed up the reaction kinetics of organic molecules.
In recent years, the group's work has been focussed on polyelctrolytes, such as different derivatives of dPG and heparin.
International Research Training Group 2662
The International Research Training Group 2662 "Charging Into The Future" (IRTG 2662) is a co-operation between Freie Universität Berlin, McGill University, and the University of British Columbia. The IRTG 2662 is funded by the Deutsche Forschungsgemeinschaft (DFG).
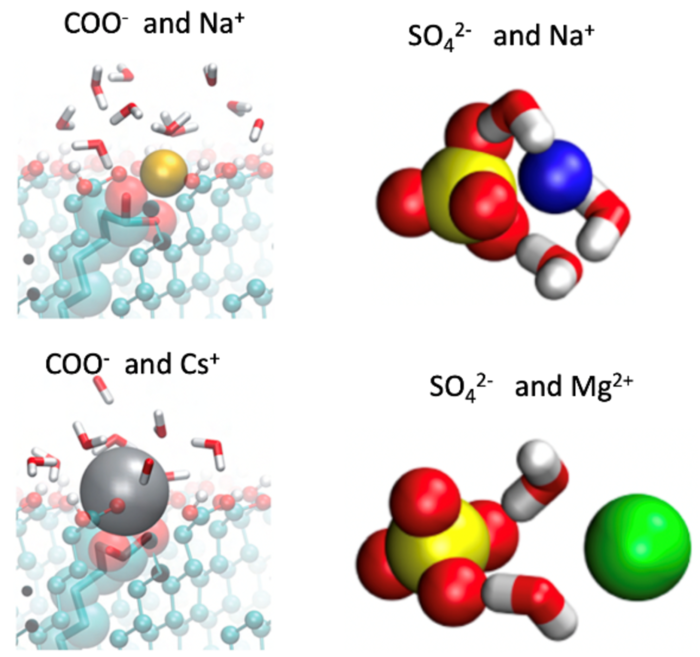
Our group's work within the IRTG 2662 is devoted to an in-depth understanding of the interaction of proteins with polyelectrolytes. Currently, there is a good understanding of the basic features of charge-charge interaction in polyelectrolyte-protein systems. However, ion-specific effects play an important role for the interaction of proteins with polyelectrolytes and are not fully understood. For example, carboxylate groups differ in their interaction with proteins as compared to sulfate groups, as illustrated in the Figure. Sulfate forms solvent-separated ion pairs with Mg2+ but not with Na+, in contrast to carboxylate, which forms a classical contact ion pair. Moreover, co- and counterions also exert an marked influence on complexation, so the problem of the interaction of proteins with polyelectrolytes is intimately related to an advanced understanding of the Hofmeister-effects.
In order to advance the molecular understanding and modelling of polyelectrolyte/protein interactions, molecular dynamics (MD) modelling will be combined with isothermal titration calorimetry and microscale thermophoresis for different temperatures and salt concentrations. First, dendritic polyglycerols with carboxyl, sulfate and sulfonate end groups will be analyzed with regard to ion-specific interactions. Here, MD-simulations will extract thermodynamic fingerprints of binding such as binding enthalpies, entropies and heat capacity changes, which can directly be compared to experimental data obtained by ITC. The overall goal of these studies is the dissection of charge-charge interaction as opposed to hydrophobic interaction and effects related to hydration in general. Modelling the binding between charged macromolecules will involve modified Poisson-Boltzmann theory that includes specific ion-protein interactions and molecular-dynamics simulations that account for hydration effects and use state-of-the-art force fields for sulfate ions and water-soluble polymers. Precise measurements of the activity coefficients of counterions will be obtained and compared to the results obtained by simulations. In the second step, the interaction of more complex macroions and their interaction with proteins will be studied. In particular, a fully quantitative understanding of the universal heparin reversal agents (UHRA) used in project C1 should be developed using a combination of MD-simulations with precise ITC-studies. The ultimate goal of these combined efforts is the full understanding of the interaction of a complex polyion with proteins and natural polyelectrolytes as heparin in a highly complex environment as blood.
Collaborations
At FU Berlin, we collaborate with various groups, including those of Dr. Marina Pigaleva (Biophysical Chemistry), Prof. Dr. Thomas Risse (Physical Chemistry), Prof. Dr. Kevin Pagel (Organic Chemistry), Prof. Dr. Daniel Lauster (Biopharmaceutics), Prof. Dr. Daniel Klinger (Medicinal and Pharmaceutical Chemistry), and Prof. Dr. Roland Netz (Physics).